Glass types
The various pharmacopoeia classify the types of glass (e.g. European Pharmacopoeia, Chapter 3.2.1 "Glass Containers for Pharmaceutical Use").
Neutral glass is a borosilicate glass containing significant amounts of boric oxide, aluminium oxide alkali and/or alkaline earth oxides. Due to its composition neutral glass has a high hydrolytic resistance and a high thermal shock resistance.
Soda-lime-silica glass is a silica glass containing alkali metal oxides (mainly sodium oxide) and alkaline earth oxides (mainly calcium oxide). Due to its composition, soda-lime-silica glass has only a moderate hydrolytic resistance.
Typical composition
| Type I | Type II | Type III | |
| Injectable | Injectable | Drinkable | |
| Silicium SiO2 | 65-72 % | 70 % | 70% |
| Soda Na2O | 5-9 % | 15 % | 15% |
| Limestone CaO | 0-4 % | 10 % | 10% |
| Borax B2O3 | 14 % | - | |
| Overige | 8-13 % | 5% | 5% |
Amber glass: colouration achieved by adding iron oxide (Fe2O3)
Classification of glass containers
The hydrolytic stability of glass containers for pharmaceutical use is expressed by the resistance to the release of soluble mineral substances into water under the prescribed conditions of contact between the glass and water. The hydrolytic resistance is evaluated by titrating released alkali.
According to their hydrolytic resistance, glass containers are classified as follows:
- Type I glass containers: Neutral (borosilicate) glass, with a high hydrolytic resistance due to the chemical composition of the glass itself.
- Type II glass containers: Usually of soda-lime-silica glass with a high hydrolytic resistance resulting from suitable treatment of the inner surface (see below),
- Type III glass containers: usually of soda-lime-silica glass with only moderate hydrolytic resistance.
Glass characteristics:
- Excellent chemical resistance; not inert!
- Impermeable to gases
- Resistance to thermal shock
- Resistance to heat
- Easily cleaned, sterilized & depyrogenated
- Transparent or coloured
- Rigid, strong, dimensionally stable
- Suitable for all types of pharmaceuticals
- Hydrolytic Resistance.
Typical applications
Although the manufacturer of a pharmaceutical product is responsible for ensuring the suitability of the chosen container, we typically recommend the following:
- Type I glass containers are suitable/preferred for most preparations for (human) parenteral use.
- Type II glass containers are suitable for most acidic and neutral aqueous preparations for (veterinarian) parenteral use
- Type III glass containers are in general suitable for:
- non-aqueous preparations for parenteral use,
- for powders for parenteral use (except for freeze-dried preparations) and
- for non-parenteral preparations
As highlighted, glass containers with a hydrolytic resistance higher than that recommended above for a particular type of preparation may generally also be used.
Surface treatments
The outer surface may be treated, for example to reduce friction and to improve resistance to abrasion; the so-called hot-end (metallic oxide deposition) and cold-end (application of a lubrication layer) treatments. For more information click here..
The inner surface of glass containers may be specially treated to improve the hydrolytic resistance through the interdiffusion/ion-exchange of sodium ions out of the glass, along with the subsequent reaction of the sulphate species with available sodium at the surface to form sodium sulphate.
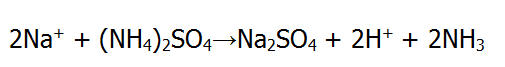
The sodium sulphate is left behind as a water-soluble crystalline deposit on the glass surface that must be rinsed away prior to filling.







